Soft robots offer numerous advantages, including integrated actuation, flexibility, compact size, lightweight structure, environmental adaptability, and low noise. These characteristics enable them to navigate unstructured environments and perform complex tasks, demonstrating immense potential in the field of medicine, human-machine interaction, and disaster rescue. However, the design, functionalization, and fabrication of soft materials remain complex. Integrating multiple responsive properties effectively to construct self-actuating, biocompatible soft robots for medical and pharmaceutical applications remains a significant challenge.

Recently, Professor Wenwen Huang’s research team at Zhejiang University published a back cover article in Advanced Healthcare Materials. The study proposes a bioinspired strategy that integrates genetic engineering and chemical engineering to construct programmable, self-actuating protein hydrogel soft robots capable of complex spatial deformation. The paper has been featured on several science communication platforms, such as NetEase, MaterialsViews, and Hydrogel Science.
Inspired by post-translational modifications in natural proteins, the research employs a multidisciplinary approach involving genetic engineering, materials chemistry, and multiscale simulations to design, fabricate, modify, and optimize recombinant protein hydrogels with specific stimulus-responsive properties from the molecular level in a bottom-up manner. As shown in the figure below, the authors used a genetic engineering strategy to design recombinant silk-elastin-like protein hydrogels with customizable stimulus responsiveness. Additionally, chemical engineering served as a secondary control point to specifically modulate the physicochemical properties of tyrosine side chains, enabling patterning and reprogramming of the stimulus-responsive hydrogel’s actuation behavior.
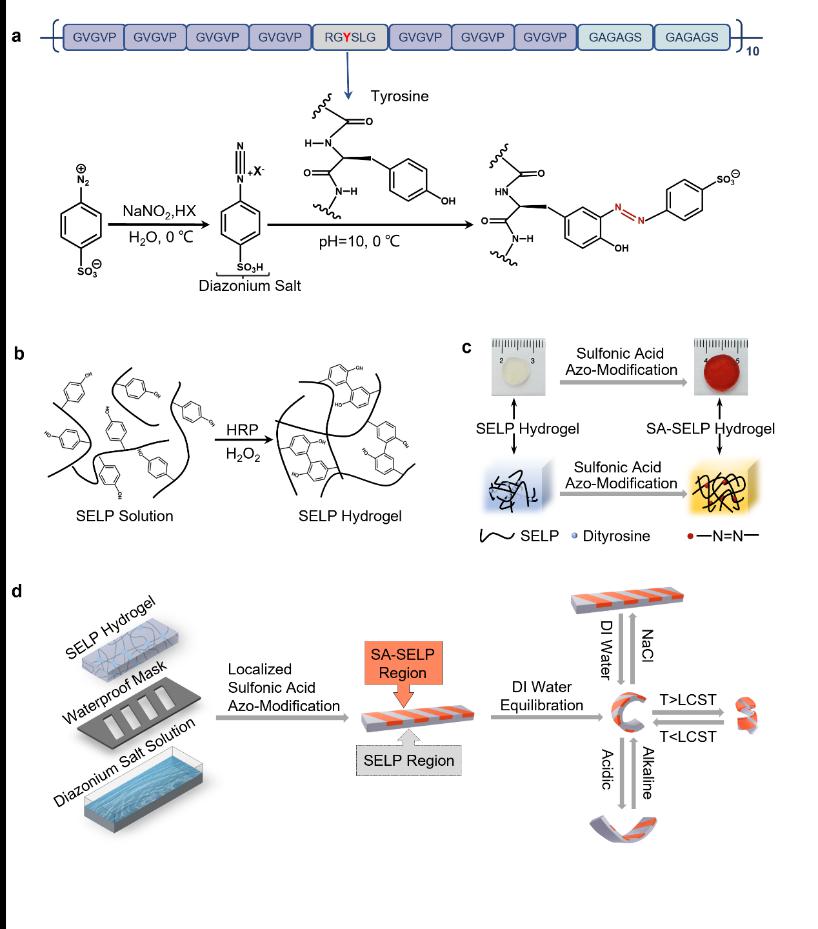
The protein hydrogel soft robots developed in this study exhibit preprogrammed, controllable environmental-responsive actuation behaviors, such as bending/unfolding and coiling/uncoiling. These behaviors are driven by asymmetric swelling induced by temperature changes (via LCST properties) or by variations in ionic strength or pH (via zwitterionic properties).
In summary, the authors proposed a strategy to modulate LCST-type and zwitterionic-type biomolecular stimulus responsiveness at the molecular level. Through the integration of genetic and chemical engineering, they controlled the topological structure of the active and passive layers within the soft robots. This approach effectively prevented layer separation and enabled the efficient fabrication of programmable, self-actuating soft robots. The strategy is highly customizable, adjustable, easy to process, and flexible, making it a promising solution to meet the demands of biomedical materials and medical robotics.
This work was led by Ph.D. student Ting Ji from Wenwen Huang’s team at ZJE, who has since graduated and joined Northwest Normal University as faculty member. Professor Huang is the sole corresponding author of the paper. The research was supported by grants from the National Natural Science Foundation of China and the Zhejiang Provincial Natural Science Foundation.
Article link: https://onlinelibrary.wiley.com/doi/10.1002/adhm.202470175







