CD8+ T cells play central roles in adaptive immune responses, utilizing their T cell receptors (TCRs) to recognize antigens associated with infection, inflammation, and cancer, exhibiting cytotoxic functions [1-2]. However, CD8+ T cells display significant phenotypic heterogeneity across various psychological and pathological conditions [3]. The rapid advancement of single-cell immune profiling technologies has resulted in the availability of millions of single-cell T cell datasets, along with paired TCR information [4]. Integrating these datasets to create a comprehensive reference atlas of human CD8+ T cells is essential for advancing our understanding of their diverse roles in immunity.
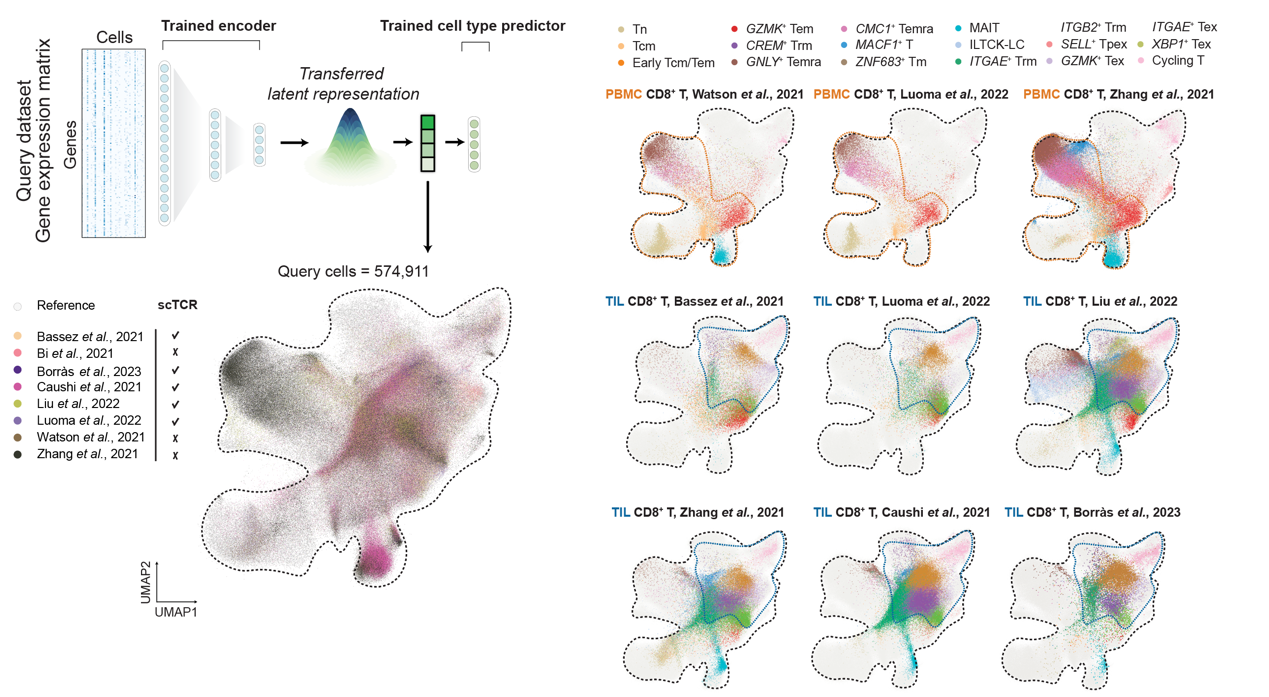
Liu Wanlu's lab from ZJE, in collaboration with Lu Linrong’s Lab from the School of Medicine, Zhejiang University, and Researcher from Tencent AI Lab, has published an article titled "Integrative mapping of human CD8+ T cells in inflammation and cancer" in Nature Methods. They developed scAtlasVAE, a single-cell integration method tailored for atlas-level scRNA-seq data. Using this method, the research team established a pan-disease human CD8+ T cell atlas encompassing 1,151,678 cells, deriving from 961 samples across 68 studies, and covering 42 distinct disease conditions. Most importantly, each CD8+ T cells were accompanied by TCR information. This atlas not only comprehensively uncovers the heterogeneity of CD8+ T cells but also provides robust support for a deeper understanding of their roles in various disease contexts.
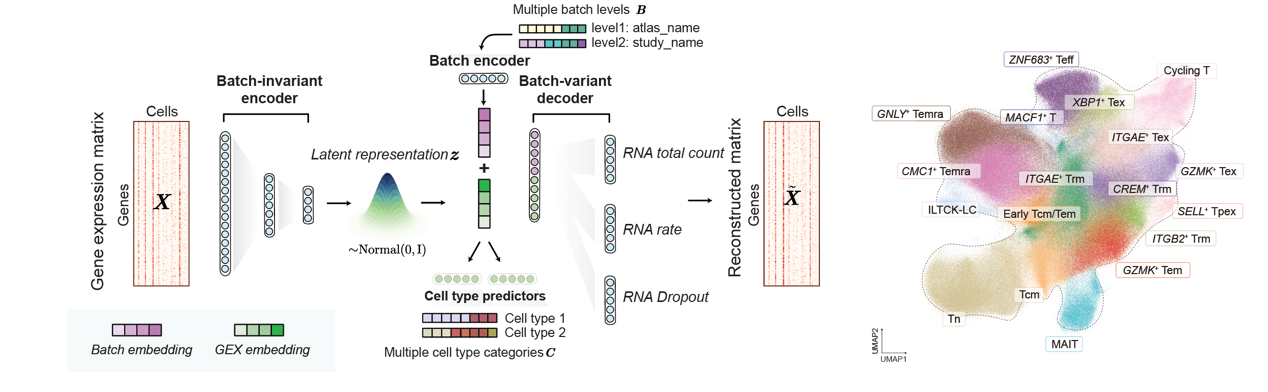
Utilizing this reference atlas, the research team conducted a comprehensive and in-depth analysis of the phenotypic heterogeneity of human CD8+ T cells in cancer and inflammation. By integrating TCR clonal expansion and sharing information, they unveiled potential connections among different subtypes and discovered their potential phenotypic and functional transitions. For instance, their approach successfully characterized three distinct exhausted CD8+ T cell subtypes. These subtypes exhibited unique transcriptome features and clonal sharing patterns in cancer, autoimmune inflammation, and immune-related adverse events (irAEs).
Their integrated pan-disease human CD8+ T cell atlas, coupled with the scAtlasVAE model, provides invaluable resources for scientists and clinical researchers. When combined with other reference atlases, scAtlasVAE emerges as a powerful tool for researchers to integrate atlas-scale scRNA-seq data and perform cross-atlas comparisons. As our atlas integrates CD8+ T cell data from a variety of disease conditions, it achieves a balance between functional relevance and generality.
Unraveling the functional diversity of the TCRαβ repertoire will be crucial for further understanding the functional heterogeneity of CD8+ T cells and their associations with various diseases. Additionally, in-depth analysis of CD4+ T cells, cross-species comparisons of T cells at the single-cell level, and integration of spatial interactions between T cells and other cell types will all provide new perspectives and insights into our understanding of the functional heterogeneity of T cells under physiological and pathological conditions.
Prof. Linrong Lu from the Zhejiang University School of Medicine, Dr. Wanlu Liu from the Zhejiang University-Edinburgh University Joint Institute, and Chief Scientist Jianhua Yao from Tencent AI Lab are the corresponding authors of this article. PhD student Ziwei Xue from the Zhejiang University-Edinburgh University Joint Institute and PhD student Li Ze Wu from the Zhejiang University School of Medicine are the co-first authors of this work. Researchers Bing He and Yu Zhao from Tencent AI Lab, along with undergraduate students Bingkang Zhao and Yicheng Li from the 2021 Bioinformatics program, also contributed to this project. The project received strong support from Professor Lie Wang of the Zhejiang University School of Medicine and was funded by the National Natural Science Foundation of China and Tencent AI Lab's Rhino-Bird Research Program.
Biographical Information of Co-First Authors Ziwei Xue and Li Ze Wu:
Ziwei Xue graduated with a Bachelor's degree in Biomedical Sciences from the ZJE in 2017 and was subsequently admitted to the dual doctoral program in Bioinformatics under Professor Wanlu Liu's lab at ZJE. His research focuses on single-cell and spatial multi-omics, investigating the pathogenesis and potential therapeutic strategies of T cells in tumors and autoimmune diseases. He has participated in the development of multiple algorithms and databases, with results published as first author (including co-authorship) in journals such as Nature Methods, Nucleic Acids Research, and iScience.
Li Ze Wu also graduated with a Bachelor's degree in Biomedical Sciences from ZJE in 2017. He continued directly to doctoral studies at the Zhejiang University School of Medicine, receiving interdisciplinary training in immunology and bioinformatics under Professors Linrong Lu and Wanlu Liu. His doctoral research focuses on the construction, analysis, and clinical application of a human antigen receptor database. His main research areas include the mapping and analysis of large-scale lymphocyte-antigen receptor data, as well as the exploration of T cell subtypes and functional mechanisms within clinical data. He has published research papers as first author (including co-authorship) in Nature Methods and Nucleic Acids Research.
Biography of Prof. Linrong Lu:
Prof. Linrong Lu's team primarily studies T cell biology and the mechanisms underlying autoimmune diseases. Previous research has revealed new mechanisms of TCR signaling enhancement in the positive selection of thymic T cells and identified new pathways for the regulation of Th cell differentiation. They were also among the first to apply CAR-T technology to the treatment of autoimmune diseases. Prof. Lu has published over 70 academic papers in prestigious journals such as Nature Immunology, Immunity, Nature Methods, JEM, PNAS, NAR, and BLOOD. He has received funding from the National Natural Science Foundation for Distinguished Young Scholars etc.. He is an Honorary Professor at the University of Edinburgh and serves as Vice President of the Immunology Cell Branch of the Chinese Society for Cell Biology and a Standing Committee Member of the Infection Immunology Branch of the Chinese Immunology Society. He is also on the editorial board of journals such as Cellular and Molecular Immunology and Oxford Open Immunology.
Biography of Researcher Wanlu Liu:
Wanlu Liu's team focuses on bioinformatics and computational immunology, employing single-cell spatial multi-omics and deep learning algorithms. As corresponding author, she has published 44 research papers in journals such as Cell, Nature Methods, Nature Communications, and Nucleic Acids Research. She has applied for three national invention patents (one granted) and leads projects funded by the National Natural Science Foundation and Tencent AI Lab's Rhinoceros Bird Special Research Program. She has been recognized as a provincial and national young talent and holds various academic positions, including member of the Immunology Cell Biology Branch of the Chinese Society for Cell Biology, director of the Zhejiang Bioinformatics Society, member of the Youth Professional Committee of the Zhejiang Bioinformatics Society, and Honorary Lecturer at the University of Edinburgh.
Zhang, N. & Bevan, M. J. CD8+ T cells: foot soldiers of the immune system. Immunity35, 161–168 (2011).
Collier, J. L., Weiss, S. A., Pauken, K. E., Sen, D. R. & Sharpe, A. H. Not-so-opposite ends of the spectrum: CD8+ T cell dysfunction across chronic infection, cancer and autoimmunity. Nat. Immunol. 22, 809–819 (2021).
Koh, C.-H., Lee, S., Kwak, M., Kim, B.-S. & Chung, Y. CD8 T-cell subsets: heterogeneity, functions, and therapeutic potential. Exp. Mol. Med. 55, 2287–2299 (2023).
Pai, J. A. & Satpathy, A. T. High-throughput and single-cell T cell receptor sequencing technologies. Nat. Methods 18, 881–892 (2021).







